Effect of magnetic water treatment on homogeneous and heterogeneous precipitation of calcium carbonate
In this paper are reported experimental results on the effect of a magnetic field on the precipitation process of calcium carbonate scale from a hard water. Carbonically pure water was circulated at a constant flow rate in a magnetic field. After this treatment, calcium carbonate precipitation was induced by degassing dissolved carbonic gas. The nucleation time was identified from the variations of the pH and the Ca2+ concentration. The ratio between homogeneous and heterogeneous nucleation was determined from the measurement of the mass of precipitated calcium carbonate.
It is shown that the magnetic treatment increases the total amount of precipitate. This effect depends on the solution pH, the flow rate and the duration of the treatment. In addition, the magnetic treatment modifies the ratio between homogeneous/heterogeneous nucleation. Homogeneous nucleation is promoted by an increasing the pH of water, the flow rate as well as the residence time. The magnetic treatment enhances these effects with a maximum for a 15 min treatment time. It is shown that the presence of calcium carbonate colloid particles is not necessary. It is advanced that the main magnetic effects concern the associations of ionic species which are present in the solution and which are involved in the nucleation process of calcium carbonate precipitation
1 Introduction
The antiscale magnetic treatment (AMT) of hard waters is currently used to prevent the build up of deposits on the walls of industrial systems, in particular in heat exchangers, as well in domestic equipments (Proceedings of the International meeting of Antiscale magnetic treatment, 1996; MAG 3, 1999). This kind of physical treatment presents the great advantage to avoid the use of chemicals such as strong acids or polyphosphates which are expensive and can be harmful for human life or deleterious for the environment. AMT has been employed for more than a half century. The first commercial
device was patented in Belgium in 1945 (Vemeiren, 1958). Powerful electromagnets were used in hot water systems since the 1960s in the Soviet Union (Grutsch, 1977). The application of AMT was reported in the United States since 1975 (Grutsch, 1977; Grutsch and McClintock, 1984). In many cases, the field is delivered by permanent magnets in various geometrical configurations. Several devices are based on AC or pulsed fields. (Oshitani et al., 1999). According to the review paper of Baker and Judd (1996), in spite of this long experience, the efficiency of this treatment is still a con- troversial question and it is not possible to get a clear explanation of the phenomenon.
A great number of experimental researches were carried out on the modification of the CaCO3 precipitation process by AMT. In most cases, a supersaturated solution was prepared by the ‘‘double decomposition method’’, by mixing two equimolar solutions of CaCl2 and Na2CO3, respectively, but this method presents the drawback to introduce systematically an excess of foreign Na2+ and Cl— ions which increase the conductivity. Various electrochemical and non-electrochemical techniques were used to estimate the scaling potentiality of water (Hui and Le´dion, 2002). In the electrochemical methods, the strong increase of the interfacial pH on the cathode plate due to the reduction of dissolved oxygen induced the heterogeneous nucleation of calcium carbonate. The scaling rate can be evaluated from the decrease of the electrochemical current intensity (Le´dion et al., 1985), by measuring the deposited mass thanks to a quartz crystal microbalance (Gabrielli et al., 1996b), or even by performing electrochemical impedance analysis (Gabrielli et al., 1996a; Deslouis et al., 1997). Among the non-electrochemical methods, some of them consist in forcing the calcium carbonate precipitation by increasing the pH, some other are based on the heating or the evaporation of the solution (Euvrard et al., 1997).
According to the literature, the efficiency of the magnetic treatment depends on numerous parameters. For example, Chibowski et al. (2003) or Barrett and Parsons (1998) have observed that a magnetic treatment applied during on a hard water decreased the quantity of scale deposited on the wall. The principle of the phenomenon is still not well understood and various contradictory hypotheses were proposed. It was often attributed to the Lorentz forces ~F ¼ q • ~v ~ ~B exerted
In a previous work (Gabrielli et al., 2001), the scaling power of a magnetically treated water was evaluated by mean of an electrochemical method. The crystal growth rate was directly measured with an electrochemical quartz crystal microbalance. It was shown that the field strength, the composition and the flow velocity of the water have significant effects on the scaling rate. The nature of the material of the tubing, in which the MF was applied, appeared also to have an influence on AMT. It was deduced that electrokinetic phenomena occurring in the vicinity of the walls of the tubing could be also implied (Gabrielli et al., 2001). However, it could be objected that the electrochemical precipitation test induces exclusively an heterogeneous nuclea- tion by a strong interfacial pH shift, this could be in contra- diction with what happens in usual conditions where the homogeneous and heterogeneous precipitation are mainly due to the escape of carbonic gas from the water, under the effect either of a change of pressure or an increase of the temperature. For this reason, the experimental works which are presented in this paper were performed with the same magnetic treatment system which proved its efficiency, but the scaling potentiality of the treated water was evaluated by inducing the calcium carbonate precipitation by a method based on the extraction of dissolved carbonic gas. From the measurement of the variations of the solution pH, the Ca2+ concentration, and the mass of the homogeneous precipitate, it was possible to evaluate the effect of the magnetic treatment on the nucleation time and on both the homogeneous and heterogeneous precipitation rates. The morphology of the two kinds of precipitate was examined by SEM. Their crystal structure was identified by X-ray diffraction.
2 Experimental
2.1 Water preparation
In order to avoid any side effect by foreign ions, calcocarbo- nically pure water (CCP water), (water containing only Ca2+,CO2— and HCO— ions) was used. It was prepared by dissolvingenthalpy of calcium carbonate formation would be modifiedby the MF (Ferreux, 1992). The scale modification could also result from the preferential formation of the aragonite polymorph (Coey and Cass, 2000; Knez and Pohar, 2005; Kobe et al., 2001), which is usually formed above 60 1C, instead of calcite. Aragonite, which may result from the transformation of metastable vaterite nuclei (Gabrielli et al., 1999), exhibits a characteristic needle shape morphology with a rather weak adhesion to the substrate. Therefore, they could be carried away by the liquid flow (Kronenberg, 1985). On the contrary, calcite which is the more stable calcium carbonate poly- morph at room temperature (Plumber and Busenberg, 1982), forms dense and tenacious layers, which are difficult to remove mechanically. It was advanced that the magnetic effect concerns ferromagnetic impurities which are nuclea- tion seeds (Busch and Busch, 1997). Lundager Madsen has concluded that the field accelerates the crystallization of sparingly soluble diamagnetic salts of weak acids such as 0.3 to 0.5 g dm—1 reagent grade CaCO3 in deionized water, by bubbling carbon dioxide during a full day according to CO2 þ H2O þ CaCO3 ! Ca2þ þ 2HCO3—: (1)
This dissolved CaCO3 quantity is equivalent to a water hardness of 30–50 French Degrees (1 1F corresponds to 10 mg dm—3 of dissolved CaCO3). This hardness can be considered as moderate by comparison with the values used by many authors (Chibowski et al., 2003; Higashitani et al., 1993; Higashitani and Oshitani, 1998; Knez and Pohar, 2005). The water pH resulting from this preparation was about 5.7. Then, the calcocarbonic equilibrium was displaced towards supersaturation by adjusting the pH between 6 and 7.5 thanks to a strong stirring to exhaust a part of the dissolved CO2 in the atmosphere. The supersaturation O depends on the (Ca2+) and (CO2—) activity according to ðCa2þÞðCO2—Þ O ¼ carbonates and phosphates. He suggested that MF is able to change the orientation of the proton spin and to disturb dehydration phenomena by hindering the transfer of the proton to a water molecule (Lungader Madsen, 1995, 2004).
Where Ks is the equilibrium solubility product for a given calcium carbonate polymorph, more generally for calcite, which is the most stable form.
| Table 1 – Supersaturation coefficient O versus water hardness and pH | ||||
|
Hardness (1F) pH | ||||
|
| 5.7 | 6.0 | 7.0 | 7.5 |
| 30 0.05 0.18 1.32 6.1 | ||||
| 40 0.15 0.32 3.2 10.11 | ||||
| 50 0.23 0.47 4.72 14.93 | ||||
|
| ||||
On Table 1, are reported the values of O relatively to calcite for various hardness of a CCP water at different pH. These data were calculated on the basis of the Legrand and Poirier model of the calcocarbonic system (Legrand et al., 1981). For pH ¼ 5.7 and 6, o was less than 1, which means that the water was undersaturated and aggressive with respect to solid calcium carbonate. For pH ¼ 7 and 7.5, the solution was moderately supersaturated and, theoretically, CaCO3 nucleation could spontaneously occur. But it is a matter of fact that it is necessary to reach a larger critical supersaturation Oc (generally OcE40 for homogeneous nucleation and OcE20 for hetero- geneous nucleation) to get calcium carbonate nucleation and growth. So, in the present conditions, these waters did not contain any calcium carbonate seeds, contrarily to the cases presented in the literature where the hardness was very high.
2.2 Water Treatment
A volume V ¼ 0.50 dm3 of CCP water was circulated in close loop from a thermostated closed glass tank through a plastic tubing (TygonTM R3606, section area s ¼ 0.38 cm2, total length 150 cm) thanks to a volumetric gear pump (Fig. 1a). The flow rate was moderate in order to have a laminar regime. A part of the tubing (l ¼ 20 cm) was inserted between the polar pieces of the magnetic device. This length can be doubled by passing twice the pipe between the polar pieces. For the sake of comparison, experiments were also carried out without passing in the MF. The magnetic device was described in details in the paper of Gabrielli et al. (2001): a series of 5 pairs of permanent magnets with north and south faces facing each other are associated alternately (Fig. 1b).
The magnetic circuit of each pair was closed with a U shaped iron yoke. The field strength was about 0.16 T in the air gap. The total time of treatment tT was between 5 and 30 min. In this configuration, the mean time of exposure of water to the MF texp for n passes, is independent of the flow rate j:
2.3. Precipitation test
In order to evaluate the scaling potentiality of the treated water, the precipitation of the calcium carbonate was induced by following a method adapted from the so called ‘‘critical pH method’’ where the pH was slowly increased by addition of a ilute NaOH solution (Feitler, 1972). As soon as the critical supersaturation was reached, the first calcium carbonate nuclei were formed and the pH began to decrease because of the liberation of H+ ions according to:Ca2þ þ HCO3— ! CaCO3 þ Hþ: (4)
The induction time tind of CaCO3 nucleation which is needed for the formation of the first nuclei, corresponds to the change of the slope of the curve pH ¼ f(t). In the present work, similar to the LCGE method (Laboratoire Chimie et Genie de l’Environnement : Dedieu et al., 1994; Elfil and Roques, 2001), the pH variations are induced by exhausting
CO2 from the water according to the reverse of Eq. (1). This method, which does not need any addition of chemicals, gives a good simulation of most natural precipitation processes where scaling phenomena are due to CO2 depar- ture.
In the precipitation test set-up (Fig. 2), 0.5 dm3 of treated water was placed in a cell made of a dense polyamide material and immersed in a thermostatic water bath to maintain the temperature at 30.0 1C. It was shown that this polyamide material exhibits a good affinity for the CaCO3 heterogeneous precipitation (Ben Amor et al., 2004). CO2 degasification was provoked by a continuous flow of pure nitrogen (8 dm3 min—1) through a diffuser located at the bottom of the cell. The solution pH and the conductivity were continuously monitored to detect the nucleation time. In a first approximation, the conductivity is proportional to the water hardness. Minute water samples (1 cm3) were drawn out every minute and their calcium ion concentration was analysed by EDTA complexometry. (accuracy E0.10—3 M). For example, Fig. 3 presents the typical variations of the pH and the calcium concentration versus time. The nucleation time tind evaluated from the two curves are in good agree- ment. In the present example, the critical supersaturation was reached at pHE8.5 and the induction time was equal to
10.570.5 min. The variations of the conductivity gave similar results. At the end of the treatment, the full volume was filtered with a 0.45 mm pore size cellulose nitrate membrane to separate and to weight the calcium carbonate mass mb formed in the bulk of the solution by homogeneous nuclea- tion. The mass of calcium carbonate mw deposited on the walls of the cell by heterogeneous nucleation is deduced from the values of mb and [Ca2+]. The morphology of the calcium carbonate grains formed in the solution or on the wall was studied by SEM and their crystal structure was determined by X-ray diffraction.
3. Results
3.1 Effect of the magnetic treatment on the induction time In preliminary experiments, it was verified that the circula- tion of the CCP water in the plastic tubing has no significant effect on the pH and on the Ca2+ concentration, whatever the solution pH and the flow rate. This result is valid in the absence as well in the presence of MF on a part of the plastic tubing. The pH increase observed between the beginning and the end of the magnetic treatment did not exceed 0.1 units for a 15 min processing time and it was about 0.2 for 30 min. It means that the treatment does not induce any significantCO2 departure due to water agitation, and that no CaCO3 particles can be formed during the treatment time.
In Fig. 4 are reported a set of experimental induction times measured during the precipitation process, as evaluated by the variations of the pH and the calcium concentration. In all cases, the water hardness was 40 1F, the temperature 30 1C and the total treatment time was 15 min, in the presence or absence of MF. The flow rate was varied from 0 to
0.94 dm3 min—1 and the solution pH from 6 to 7.5. This figure demonstrates clearly that these three parameters: flow velocity, initial pH, and application of the MF tend to reduce the induction time. It is equivalent to say that they increase the calcium carbonate nucleation rate. The effect of pH is easily understandable: when the water becomes more alka- line, the calcocarbonic equilibrium is displaced towards a stronger supersaturation and the nucleation probability becomes larger. However, the influence of the flow rate in the absence of MF is somewhat surprising. It is worth noting that the simple circulation of the water in the tubing has a effect on the induction time of CaCO3 nucleation measured thereafter during the precipitation test. For example, for an initial pH of 6, tind decreased from 22 to 12 min when the flow rate varied from 0 to 0.94 dm3 min—1. This effect was less evident when the solution pH was higher. In our knowledge this effect had never been mentioned until now. When the MF was applied along 20 cm of the plastic pipe, the induction time was even more reduced and this effect increased with the flow velocity.
In Table 2, are illustrated the influence of the processing time on the induction time in the presence of MF, for 2 pH values and for various flow rates in the case of a 40 1F water hardness. One can observe that in all cases, there was an optimum treatment time of about 15 min, corresponding to the smaller induction time—or the higher nucleation rate. For a longer treatment time, paradoxically the effect of the MF was less pronounced.
Fig. 1 – Sketch of the set-up for the magnetic treatment of circulating water. (a) set up and (b) permanent magnets disposition.
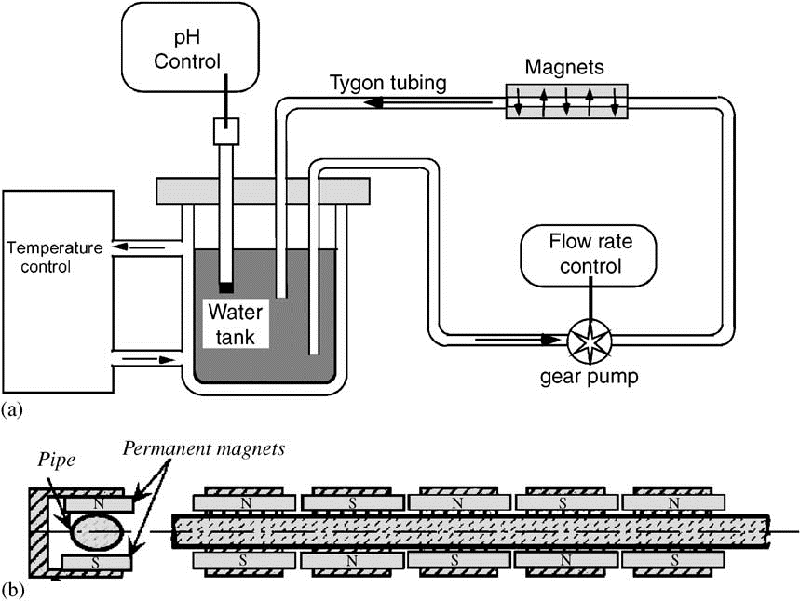
Fig. 2 – Scheme of the set-up for the precipitation test by CO2 degassing
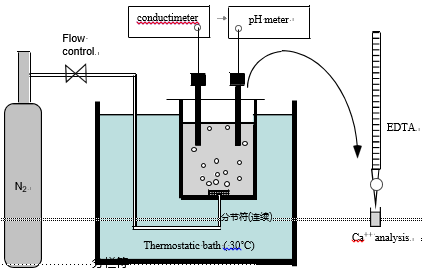
Fig. 3 – Variations of the solution pH and the calcium ion concentration during a precipitation test (H ¼ 40 1F, initial pH ¼ 7).
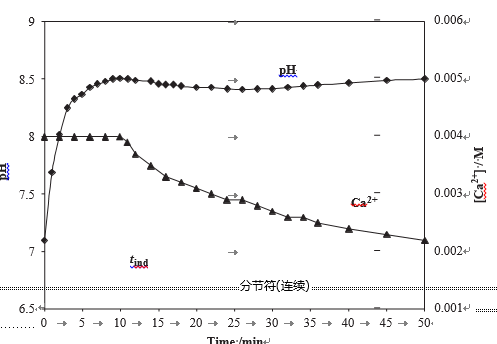
Fig. 4 – Variations of the induction time tind versus the flow rate for various initial values of the initial pH, in the presence or absence of magnetic field.
3.2. Effect on homogeneous and heterogeneous nucleation
In Fig. 5 are reported experimental measurements of the percentage of the total quantity of precipitated calcium carbonate, expressed in percentage of the initial CaCO3
quantity dissolved in the water. This quantity was deduced from the measurement of Ca2+ concentration after the treatment. These measurements were carried out on 40 1F water hardness, in the presence or not of the MF, for various flow rates, at pH 6–7.5. The treatment time was 15 min and the CO2 degassing time was 90 min. In the absence of MF, the total amount of precipitated CaCO3 did not depend signifi- cantly from the flow rate, whatever the pH. It means that the total amount of precipitate was the same although the nucleation induction time was decreased by the water circulation in the tubing. In the presence of the MF, the total amount of precipitate was significantly increased by ca. 7–22% for j ¼ 0.54 dm3 min—1, and this effect was increased even more with a higher flow rate (26% at pH ¼ 7.5 and j ¼ 0.94 dm3 min—1).
The amount of calcium carbonate precipitated in the bulk of the solution by homogeneous nucleation during the precipitation test was determined after filtration of the solution. Fig. 6 shows that the percentage of precipitated calcium carbonate by homogeneous nucleation expressed in % of the initial dissolved quantity increased with the pH and with the flow rate. When MF was applied, the amount of homogeneous precipitate was even larger. For example, in the case of a 40 1F and pH ¼ 7.0 water, at j ¼ 0.74 dm3 mn—1, in the absence of MF, the total precipitation ratio was 73% divided in 22.2% for the homogeneous precipitation ratio and 50.8% for the heterogeneous ratio. In the presence of MF, the total precipitation ratio was increased to 84%. The homogeneous contribution was increased to 38.9% whereas the heteroge- neous one was decreased to 45.1%. These experimental data give evidence that the MF promotes preferentially the homogeneous precipitation detrimentally to the scaling of the walls. This scaling inhibition confirms previous results on heterogeneous precipitation obtained by electrochemical tests (Gabrielli et al., 2001).
As illustrated by Fig. 7, the homogeneous precipitation ratio is increased by the water hardness and also by the flow rate, especially at low hardness. The specific effect of the MF is relatively more important for low hardness water.
| Table 2 – Effect of the treatment-time on the induction time at various solution pH and various flow rates | ||
|
| ||
| Flow rate Time of (dm3 min—1) treatment (min) | Induction time | |
| pH ¼ 6 | pH ¼ 7 | |
| 0.54 5 17 | 15 | |
| 15 14 | 10 | |
| 30 12 | 8 | |
| 0.74 5 14 | 9 | |
| 15 10 | 9 | |
| 30 13 | 9 | |
| 0.94 5 12 | 9 | |
| 15 7 | 7 | |
| 30 13 | 8 | |
The influence of the treatment time on the precipitation in magnetic treated water was also investigated. For a 40 1F, pH ¼ 7.0 water, Fig. 8a and b present the variations of the total and the homogeneous precipitation ratio, versus the treat- ment time, for various flow rates. They give evidence that there is an optimal treatment time (about 15 min), which corresponds to a larger total amount of precipitated calcium carbonate and a larger contribution of homogeneous pre-cipitate, whatever the flow rate.
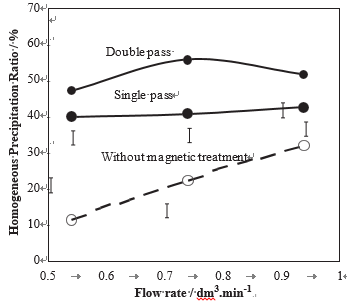
In all previous experiments, the treatment time was maintained during 15 min with a single pass between the pole pieces. That corresponds to a mean exposure time texp ¼ 0.23 min, according to Eq. (1). In order to increase the exposure time without changing the treatment time, a double pass treatment was applied. In Fig. 9, it is shown that a double pass of a 40 1F pH ¼ 7.0 water increased the % of the amount of dissolved CaCO3 versus the flow rate for treated water of various hardness (full line: in the presence of MT, dotted lines in the absence of MT, pH ¼ 7).
Homogeneous precipitation ratio from ca. 41% to ca. 52%, whereas, in the absence of MF, this ratio was only ca. 25%. This result shows that, as expected, a longer exposure time to MF increased the total amount of precipitate but also it modified the property of the treated water, in such a way that the homogeneous precipitation was preferentially increased.
Fig. 5 – Variations of the total precipitation ratio in % of the amount of dissolved CaCO3, versus the flow rate, for treated waters at various pH (full line : in the presence of MT, dotted lines in the absence of MT, H ¼ 40 1F).
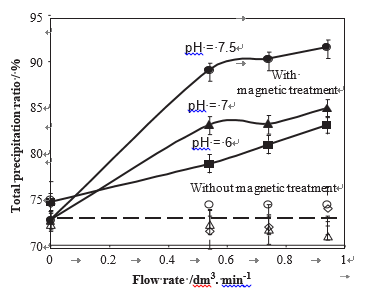
Fig. 6 – Variations of the homogeneous precipitation ratio in % of the amount of dissolved CaCO3 versus the flow rate for treated waters at two pH (full line: in the presence of MT, dotted lines in the absence of MT, H ¼ 40 1F).
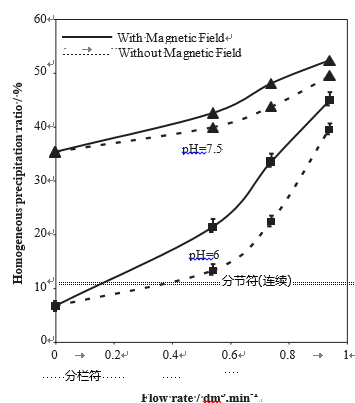
Fig. 7 – Variations of the homogeneous precipitation ratio in % of the amount of dissolved CaCO3 versus the flow rate for treated water of various hardness (full line: in the presence of MT, dotted lines in the absence of MT, pH ¼ 7).
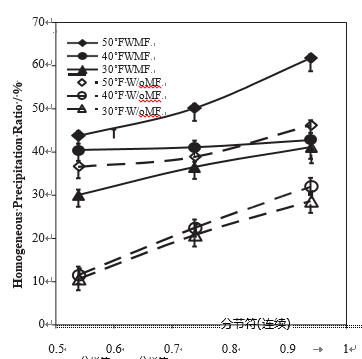
Fig. 8 – Precipitation ratio in % of the amount of dissolved CaCO3 versus the treatment time, for various flow rate (pH ¼ 7, H ¼ 40 1F). (a) total precipitation ratio and (b) homogeneous precipitation ratio.
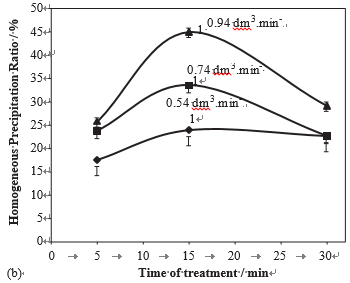
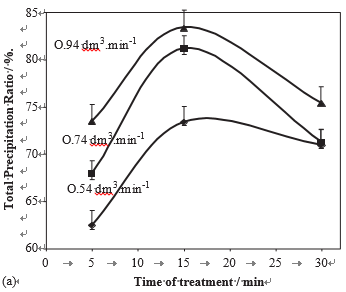
Fig. 9 – Variations of the homogeneous precipitation ratio in % of the amount of dissolved CaCO3 versus the flow rate for a treated water either in the absence of magnetic treatment or after passing one time or two time in the magnetic device (pH ¼ 7, H ¼ 40 1F).
3.3. Calcium carbonate morphology
Calcium carbonate crystals formed in the solution bulk and separated by filtration, were systematically observed by SEM. In the absence of any treatment, the crystals exhibit the characteristic morphology of the vaterite form (Fig. 10a). The identification of the crystal structure was confirmed by X-ray diffraction and also by micro Raman spectroscopy, on the basis of previous studies (Gabrielli et al., 2000, 2003).
After MF treatment; as seen in Fig. 10a and b, the formation of the calcite form is promoted. At pH ¼ 6, a part of the precipitate is made of vaterite crystals partially transformed into aragonite needles, an other part is made of calcite crystals with their characteristic near cubic shape. The mean crystal size of precipitation test was about 10 mm after 90 min of precipitation test. For a more alkaline treated water (Fig. 10c), only calcite crystals were observed, and their size was reduced to about 5 mm. Knowing that the total amount of homogeneous precipitate was increased and the nucleation time was decreased it means that the number of nuclei was significantly increased. That demonstrates that the MF had modified some species which were present in the calcareous water during the treatment, and which were implied later in the nucleation process during the precipitation test.
4. Discussion
The main findings of this experimental investigation can be summarized as follows:
* In the absence of MF, the circulation of water in the tubing has a positive effect on the calcium carbonate nucleation which is triggered later during the precipitation test.
* MF decreases the nucleation induction time and increases the nucleation rate.
* MF increases the precipitation potentiality of the treated water. This effect is more pronounced for homogeneous nucleation than for heterogeneous nucleation.
* The efficiency of the treatment increases with the water flow rate in the tubing and with the time of exposure to the MF.
The efficiency of the treatment increases with the hard- ness and with the pH of the water.
* MF treatment is efficient even for a water having a supersaturation coefficient lower than 1 (undersaturation) or having a supersaturation sufficiently small to be sure that no calcium carbonate colloidal particle be present.
* MF treatment promotes the homogeneous precipitation of aragonite and calcite crystals instead of vaterite.
Numerous mechanisms have been proposed in the litera- ture to explain the MF effects on the precipitation of calcium carbonate from hard waters. According to Knez and Pohar (2005) they are belonging to two different approaches: either magnetohydrodynamic (MHD) phenomena or hydration ef- fects.
MHD phenomena depend on the flow of the treated water. The beneficial influence of the flow rate on the magnetic effect observed in the present results is in agreement with this approach. Busch et al. (1996) have assumed that the Lorenz forces exerted on charged species induce local convection movements in the liquid which could contribute to accelerate associations between ions or colloidal particles.
In the present case, similar effects in relation with hydro- dynamic forces could be attributed to the circulation of the water. It was observed that MF promotes aggregation of latex particles only when the suspension is circulated Busch et al. (1996). According to these authors, MHD phenomena induce eddy currents which flattened the fluid velocity profile in the tube. This effect would result in a larger velocity gradient, along the walls. In addition, the streaming potential along the walls which is velocity dependent should increase with the MF. This phenomenon, by changing the surface charge, could throw out of balance the calcocarbonic equilibrium in the vicinity of the tubing walls (Knez and Pohar, 2005). MHD phenomena could also concern the electrical double layer near the charged surface of particles. This interpretation seems receivable for many experimental results of the literature where highly supersaturated waters were treated by MF (Barrett and Parsons, 1998). The aggregation of the CaCO3 colloidal particles under the influence of electrostatic phenomena would contribute to accelerate the crystal growth and the precipitation process.
However, in the present experimental conditions (CCP water, moderate pH and low hardness), we can assume that the water did not contain any calcium carbonate particles in suspension. Nevertheless, since 1941, it is known that hard waters contain various ionic associations such as ionic pairs CaCO31 (Greenwald, 1941; Plumber and Busenberg, 1982). According to Gal et al. (1996, 2002); calcium carbonate crystallization is a very complex process involving several steps: at first hydrated Ca2+ and CO2— ions are associated in CaCO31 shape. The formation of loosely attached (Ca2+—CO2—) ionic pairs weakens the water shells and hydrated micelle of disordered pairs are formed, whose complexity increases with the supersaturation. Their progressive dehydration leads to CaCO3 solid forms. In agreement with several authors (Gal et al., 1996; Elfil and Roques, 2001; Tlili et al., 2001, 2003) the solid forms which are formed at first are made of hydrated amorphous or hexagonal calcium carbonate crystal forms, so these unstable hydrated forms can be considered as the precursors of the three usual anhydrous polymorphs—na- mely calcite vaterite or aragonite. We can assume that the hydrodynamic forces as well as the electrokinetic phenomena along the walls could favour the process of ionic pair micellization. This hypothesis is supported by the fact that, after a simple water recirculation in the absence of MF, the induction time for calcium carbonate nucleation was sig- nificantly reduced during the precipitation test. So it can be supposed that the micellization of ionic pairs was more advanced, thus facilitating the effective nucleation process during the following precipitation test. In the presence of MF, these effects could be expected to be enhanced.
MF effects were also often explained in terms of magnetically induced changes in the hydration of ions or solid
surfaces. According to Lungader Madsen (1995, 2004) MF induces faster proton transfer from hydrogen carbonate to water (reaction 3), due to proton spin inversion in the field of diamagnetic salts. The increased formation of CO2— ions would explain the beneficial effect of MF on the amount of precipitate. Another explanation proposed by Higashitani et al. (1993) is related to the specific influence of MF on the hydration of CO2— ions which could directly modify the polymorph phase equilibrium during the precipitation. The same phenomenon could also affect the dehydration process of the ionic pair associations and the hydrated calcium carbonate forms which act as precursors for the formation of the anhydrous forms which constitute the calcium carbonate precipitate.
5. Conclusion
The present results show that the circulation of water in a stationary MF reduces the nucleation induction time and increases the total amount of calcium carbonate obtained thereafter during the precipitation test by CO2 degassing. This effect is more pronounced for homogeneous nucleation. It does not need the presence of CaCO3 particles in the treated water. It can be assumed that the ionic associations which are present even in unsaturated solutions and which are involved in a complex process leading to the final formation of the anhydrous calcium carbonate crystals, are affected by MHD and electrokinetic phenomena especially along the walls or by magnetically induced changes of their hydration shell.
Fig. 10 – SEM images of calcium carbonate crystals obtained by homogeneous precipitation and filtration: (a) non treated water , pH ¼ 6, H ¼ 40 1F, vaterite crystals. (b) magnetic treated water, pH ¼ 6, H ¼ 40 1F, flow rate ¼ 0.54 dm3 min—1: calcite and vaterite transformed into aragonite and (c) magnetic treated water, pH ¼ 7.5, H ¼ 40 1F , flow rate ¼ 0.54 dm3 min—1: pure calcite.





